Thousands in the UK sue Johnson & Johnson over talcum powder cancer risks

A substantial legal action has been initiated in the UK against pharmaceutical giant Johnson & Johnson, accusing the company of knowingly selling baby powder contaminated with asbestos.
The claim, involving 3,000 individuals, centers on internal memoranda and scientific reports, which have been examined by the BBC.
The lawsuit, filed by KP Law, asserts that Johnson & Johnson (J&J) was aware as early as the 1960s that its mineral-based talcum powder contained fibrous forms of talc, as well as tremolite and actinolite, both of which are classified as asbestos and linked to potentially deadly cancers.
The court documents allege that, despite knowledge of the minerals’ direct link to cancers, J&J failed to provide warnings on the packaging of its baby powder.
Instead, the company launched aggressive marketing campaigns portraying the powder as a symbol of purity and safety, according to the lawsuit.
J&J denies the allegations, as well as any claims that it knowingly sold baby powder contaminated with asbestos.
In a statement, the firm maintained that its baby powder ‘was compliant with any required regulatory standards, did not contain asbestos, and does not cause cancer.’
The UK’s cessation of talc-based baby powder sales in 2023 mirrors the extensive litigation in the US, where numerous lawsuits have been filed, resulting in billions of dollars in damages awarded to claimants, although the company has successfully appealed some of these cases.
Lawyers for the claimants estimate that damages sought in the UK could amount to hundreds of millions of pounds, potentially rendering this the largest product liability case in British history. The claims linking talcum powder to cancer center on asbestos, a known carcinogen.
Talc, a naturally occurring mineral used in J&J talcum powders, is often mined in close proximity to asbestos deposits, with the fibrous, needle-like form of asbestos minerals being associated with cancer. Confidentiality has been urged in this matter.
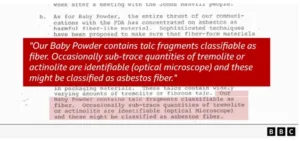
The claim alleges J&J had identified asbestos in its baby powder as early as the 1960s. One internal document from 1973 allegedly says: “Our baby powder contains talc fragments classifiable as fiber. Occasionally sub-trace quantities of tremolite or actinolite are identifiable…”
J&J says this letter was discussing how regulation might change and thereby define talc fibres as asbestos. The firm said that would have been wrong.
In the same year, executives discussed the value of a possible patent for a method that aimed to remove asbestos fibres from talc. At the end of the letter, it added: “We may wish to keep the whole thing confidential rather than allow it to be published in patent form and thus let the whole world know.”
J&J says these discussions were confidential because a new patent could have been extremely valuable if the new method had been effective. Ultimately, it did not prove to be effective.
According to the claim, the company’s marketing team deliberated on ways to boost sales despite knowing that the baby powder contained carcinogenic fibres.
In the 1970s and 1980s, US marketing concentrated on the sale of pure and gentle powder for newborn babies. The marketing focus then shifted to African American women by the 1990s and 2000s.
An internal email from 2008, obtained by the BBC, allegedly discussing branding, reads, “The reality that talc is unsafe for use on/around babies is disturbing…” The email further states, “I don’t think we can continue to call it baby powder and keep it in the baby aisle.”
J&J maintains that this conversation pertained to asphyxiation, a rare but recognized risk at the time related to the use of all body powder, and was not connected to cancer or asbestos, with warnings provided on the bottle.

The UK lawsuit cites documents allegedly showing that Johnson & Johnson executives pushed the FDA to accept lower sensitivity standards for asbestos detection in talc products from the early 1970s.
Internal documents reportedly reveal J&J’s advocacy for testing standards allowing up to 1% asbestos contamination, deeming more sensitive methods unnecessary.
This allegedly facilitated the company’s claims of product purity, misleading regulators and consumers about asbestos presence in its talc products.
J&J maintains that this misrepresents the document’s context, referencing a hypothetical FDA-requested calculation.
‘My mother used it – I used it’
Many of the claimants in the UK are suffering from, or have died from, ovarian cancer, mesothelioma – a cancer that is usually caused by asbestos exposure – or other cancers. All the claimants are alleged to have used J&J’s baby powder over an extended period of time.
“My mother used it and I used it. It smelt nice and was soft and lovely. When my babies were born I used it on them. I thought I was doing my best for them,” she told the BBC from her home in Somerset.
“It was such a shock. We just hugged and cried. I couldn’t believe what I was hearing when the doctor told me I had stage 4 ovarian cancer.”
At the time of diagnosis, it wasn’t clear how long Siobhan would survive for, but after three rounds of chemotherapy, a bout of sepsis that nearly killed her, and major surgery to her abdomen, she is alive and able to tell her story 18 months later.
Siobhan, like the other claimants in this case, thinks her cancer was caused by the use of J&J’s baby powder.
The first rounds of treatment helped control the spread of her cancer, but a few months ago Siobhan found another lump in her groin. She is now back in chemotherapy and surgeons say her cancer is no longer operable.
“They knew it was contaminated and still they sold it to new mums and their babies,” Siobhan says.
Siobhan has short pale blonde hair, black glasses and a pink jacket and sits in a brown chair in front of a potted plant.
Siobhan had used J&J’s baby powder on her children when they were babies
Ovarian cancer is caused by a combination of genetic, internal and external factors.
“The female reproductive tract is open to the external environment so that women can get pregnant,” says Prof Christina Fotopoulou, a leading gynaecological oncology surgeon at Imperial College London and a leader in the field of ovarian cancer.
“Cancer is usually an accumulation of mistakes in the reproduction cycle of the cells and so any harmful factors – internal or external – that disrupt the balance of the cells may contribute to these mistakes that eventually may lead to cancer.”
Common symptoms of ovarian cancer include persistent bloating, persistent pelvic or abdominal pain, feeling full quickly or an inability to eat, and an increased or urgent need to urinate.
Those who experience such symptoms frequently – more than 12 times a month – should see a doctor. Extreme fatigue, changes in bowel habits like constipation or diarrhoea, and vaginal bleeding after menopause are also signs you should see your GP.
Our baby powder was compliant.
Earlier this month, a court in the US state of Connecticut ordered J&J – and its successor entities – to pay $25m to a man diagnosed with terminal peritoneal mesothelioma after lifelong use of J&J baby powder. The jury in the trial found the pharmaceutical company negligent for selling asbestos-contaminated talc products.
This trial also included deposition testimony from Dr Steve Mann, former director of toxicology at J&J Consumer Products, who said he had made safety claims without reviewing any test data. Dr Mann conceded that he had received test results showing asbestos in the baby powder but chose not to inform management or regulators.
The judge noted that safer alternatives, such as cornstarch, were available and known to the company, yet J&J continued selling talc-based powder in the US until 2020 and in the UK until three years later.
Following the Connecticut judgment, J&J has denied wrongdoing and is expected to appeal.
J&J has moved its consumer health arm to a new company, called Kenvue, which said in a statement: “We sympathise deeply with people living with cancer. We understand that they and their families want answers - that’s why the facts are so important.”
It said the safety of the baby powder was backed by years of testing by “independent and leading laboratories, universities, and health authorities in the UK and around the world”.
It said J&J’s baby powder “was compliant with any required regulatory standards, did not contain asbestos, and does not cause cancer”.
source: BBC

